Engineers have been working tirelessly to make batteries cheaper, more robust and hold more energy. Li-ion batteries have been the top choice in recent years, but they are reaching the limits of their current design. A key factor which confines Li-based battery performance is dendrite formation. New research, however, indicates current understanding is only “scratching the surface.”
Li-ion batteries could hold more charge if their anodes were made from lithium instead of graphite. This is not currently feasible due to dendrite growth. As the battery is cycled (charged and discharged), microscopic pillars (dendrites) of lithium extend out from the surface and across the electrolyte causing a short-circuit, overheating and potentially a fire.
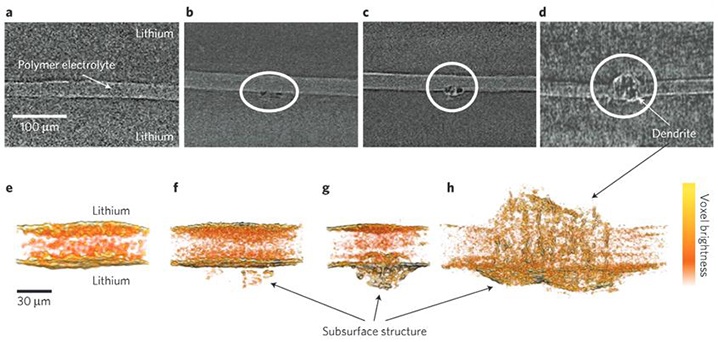
As described in Materials Views, researchers with the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) have discovered early dendrite formation is a subsurface phenomenon.
The research team, led by Nitash Balsara, a faculty scientist with Berkeley Lab’s Materials Sciences Division, observed the seeds of dendrites forming in lithium anodes and growing out into a polymer electrolyte during cycling. Observation was performed using X-ray microtomography. Unexpectedly, the dendrites did not protrude from the surface until the advanced stages of development. Balsara exaplins, “Contrary to conventional wisdom, it seems that preventing dendrite formation in polymer electrolytes depends on inhibiting the formation of subsurface dendritic structures in the lithium electrode.”
This finding indicates the limitation to lithium anodes may be overcome through addressing the nucleation rather than growth of dendrites. Again, Balsara, “In showing that dendrites are not simple protrusions emanating from the lithium electrode surface and that subsurface non-conductive contaminants might be the source of dendritic structures, our results provide a clear prescription for the path forward to enabling the widespread use of lithium anodes.”
The path forward, then, involves a look at contamination in the lithium. Impurities produce a favorable site for dendrite nucleation. Balsara and his group also plan further study of the role played by the electrolyte in dendrite growth.
A number of methods for battery improvement, such as self-healing polymers, have been employed or are in development. The demand for longer battery life in a smaller, lighter package is continually pushing engineers to think outside the box, or in this case, below the surface.

